Table of Contents
Organic chemistry, the study of carbon-containing compounds, is filled with fascinating reactions and molecules that shape pharmaceuticals, fuels, and materials around us.
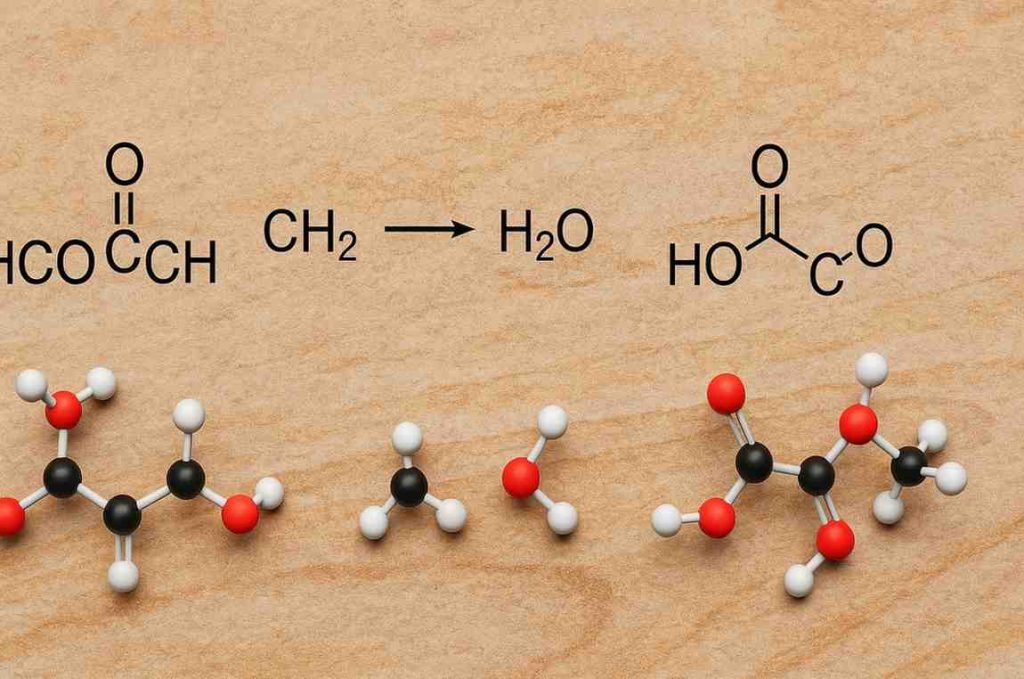
One such compound of interest in this intricate world is HCOOCH CH₂ H₂O, which combines multiple functional groups into a reactive and instructive molecule.
But what exactly is HCOOCH CH₂ H₂O? How is it formed, and why is it important in organic chemistry? This blog post unpacks its structure, reactivity, synthesis routes, and real-world applications to help you understand why this compound deserves your attention.
Understanding the Components: What Is HCOOCH CH₂ H₂O?
To truly grasp the significance of HCOOCH CH₂ H₂O, we need to break it down.
This formula suggests a molecule formed through the hydration or hydrolysis of certain esters or aldehydes.
Though it’s not a conventional chemical notation in scientific literature, it can be interpreted as a reaction system or intermediate, where:
- HCOOCH refers to methyl formate (formic acid methyl ester),
- CH₂ indicates a methylene group, commonly involved in further reactions,
- H₂O signifies the presence or addition of water, which hints at hydrolysis or a hydration step.
This mixture of symbols represents a chemical context involving methyl formate, an alkene or aldehyde, and water, which is often part of reaction mechanisms in organic synthesis.
Methyl Formate (HCOOCH₃) – The Ester Backbone
Let’s start with methyl formate (HCOOCH₃), the ester formed from formic acid (HCOOH) and methanol (CH₃OH). It’s a simple ester but has interesting uses and roles:
Properties of Methyl Formate:
- Molecular formula: HCOOCH₃
- Boiling point: 31.5°C (very volatile)
- Colorless liquid with a pleasant odor
- Easily soluble in alcohol and ether
Industrial and Laboratory Use:
- Used as a solvent, fumigant, and intermediate in organic synthesis.
- Its reactivity with water is crucial in understanding hydrolysis and esterification.
- In certain conditions, it can release CO (carbon monoxide), making it useful in CO-releasing molecule (CORM) chemistry.
The Role of Water (H₂O) in Hydrolysis
When H₂O interacts with an ester like HCOOCH₃, a hydrolysis reaction can occur, particularly in the presence of an acid or base catalyst.
Hydrolysis Reaction:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This reaction demonstrates the reversibility of esterification and is a key process in:
- Biochemistry (breakdown of fats and esters)
- Green chemistry (non-toxic degradation)
- Drug design (controlling drug release through hydrolysis)
So, in the context of HCOOCH CH₂ H₂O, water plays a dual role:
- As a reactant that breaks down esters.
- As a solvent that allows polar and ionic reactions to proceed efficiently.
What Does CH₂ Represent?
CH₂, or a methylene group, can play many roles in organic reactions. It can be:
- A linker in molecular chains,
- Part of a vinyl group (CH₂=CH–) in alkenes,
- An intermediate in rearrangement reactions,
- Involved in dehydration-condensation reactions.
For Example:
If CH₂ is part of a vinyl ester, such as methyl vinyl formate, it becomes more reactive:
CH₂=CH–O–COH (Vinyl formate)
In this state, CH₂ participates in polymerization, addition, or nucleophilic substitution, contributing to materials science and polymer chemistry.
So, HCOOCH CH₂ H₂O might also represent a vinyl ester system undergoing hydrolysis or nucleophilic addition with water.
Key Reactions Involving HCOOCH CH₂ H₂O
Now, let’s discuss some possible organic reactions involving the compound system implied by HCOOCH CH₂ H₂O.
Acid-Catalyzed Ester Hydrolysis
In the presence of acid (like HCl), HCOOCH₃ reacts with water:
HCOOCH₃ + H₂O ⇌ HCOOH + CH₃OH
This is a fundamental mechanism in both industrial ester breakdown and biochemical digestion of esters.
Aldol-Type Reactions
If the CH₂ group is part of an aldehyde (e.g., formaldehyde or acetaldehyde), then under base conditions, aldol condensation can occur, especially with a neighboring ester.
Vinyl Ester Hydrolysis
A vinyl ester (like CH₂=CH–O–C(=O)H) reacts with water and acid to yield:
CH₂=CH–OH + HCOOH, though vinyl alcohol tautomerizes to acetaldehyde.
These reactions form the basis of polymer degradation and are key in material recycling and green chemistry.
Applications in Modern Organic Chemistry
Despite its simplicity, the interaction between esters, water, and methylene groups underpins many advanced organic systems:
Read also: Lyposingrass
Pharmaceutical Synthesis
- Esters like methyl formate are used to build prodrugs.
- Controlling ester hydrolysis controls drug activation timing.
Polymer Science
- Vinyl esters, especially with methylene groups, are used in adhesives and coatings.
- Hydrolysis reactions help in controlled degradation for eco-friendly materials.
Perfumes and Flavors
- Simple esters such as HCOOCH₃ are fragrance carriers.
- Hydrolysis affects scent longevity.
CO-Releasing Molecules
- Some esters, when hydrolyzed or decomposed, release CO in small, controlled amounts—used in medical research for anti-inflammatory therapies.
Safety and Handling
Though methyl formate and its related compounds are low-toxicity, they are flammable and must be handled with care.
- Storage: Keep in cool, dry areas.
- Ventilation: Ensure good airflow to avoid vapor buildup.
- Disposal: Follow chemical disposal protocols; esters should not be poured down the drain.
Final Words
While HCOOCH CH₂ H₂O may seem like a cryptic formula at first glance, it encapsulates a web of reactions central to organic chemistry:
- Esters like HCOOCH₃ are key intermediates in synthesis.
- Water (H₂O) triggers essential hydrolysis and substitution reactions.
- CH₂ groups activate molecules for bonding and transformation.
Together, this molecular system touches everything from materials chemistry to pharmaceuticals and green technology.
Understanding it helps chemists design smarter, safer, and more efficient chemical processes.
If you’re a student, researcher, or enthusiast, mastering these fundamental interactions can enhance your grasp of how organic molecules behave and why they matter.